Janssen Vaccine Usa
The Janssen COVID-19 vaccine fact sheet for vaccine recipients and caregivers has been revised to include that vaccine recipients should seek medical attention if they develop weakness or tingling sensations in the legs or arms difficulty walking double vision or inability to move eyes difficulty with bladder control or bowel function or difficulty with speaking chewing or swallowing. Emergency Use Authorization Status.

How The Johnson And Johnson Vaccine Is Different From The Others Dosage Efficacy Protection Technology Abc7 San Francisco
It is also known as Johnson Johnson COVID-19 Vaccine and as COVID-19 Vaccine Janssen.
Janssen vaccine usa
. 132 rows As of April 12 more than 68 million doses of the Johnson Johnson. The Janssen COVID-19 vaccine is a viral vector vaccine produced by Janssen Pharmaceutica a subsidiary of Johnson Johnson and Beth Israel Deaconess Medical Center. The Janssen COVID-19 Vaccine is administered as a single primary dose. Center Director CBER FDA stated the FDA team would take this VRBPAC recommendation under.The Embassy of the United States of America in Bogota Colombia announces the donation by the US. The United States FDA has made the Janssen COVID-19 Vaccine available under an emergency access mechanism called an EUA. JJs Janssen COVID-19 vaccine is a viral vector vaccine that requires only 1 shot. NEW BRUNSWICK NJ February 27 2021 Johnson Johnson NYSE.
March 2 2021- The US CDCs Advisory Committee on Immunization Practices Interim Recommendation for using Janssen COVID-19 Vaccine United States February 2021. Learn about safety data efficacy and clinical trial demographics. The EUA allows the Janssen COVID-19 Vaccine to be distributed in the US. The US Food and Drug Administration FDA and Centers for Disease Control and Prevention CDC have concluded their review of blood clots reported after vaccination with the Janssen COVID-19 Vaccine and concluded that its benefits continue to outweigh the risks and thus the use of the vaccine should resume in the US.
Food and Drug Administration FDA has issued Emergency Use Authorization EUA for its single-dose COVID-19 vaccine developed by the Janssen Pharmaceutical Companies of Johnson Johnson to prevent COVID-19 in individuals 18 years of age and older. Food and Drug Administration FDA but has been authorized by the FDA through an Emergency Use Authorization EUA. March 1 2021 - Irving-based McKesson announced today it began distributing the Janssen COVID-19 vaccine received from Janssen Pharmaceuticals Inc one of the Janssen Pharmaceutical Companies of Johnson. That is one of the preliminary conclusions of a study that the US National Institutes of Health NIH published Wednesday.
A booster dose should be given at least 2 months 8 weeks after the initial primary dose. The Janssen COVID-19 Vaccine is an unapproved vaccine. The study has not yet been reviewed by other. During the second day of the 169th US.
US president Joe Biden confirmed on Monday that any vaccination approved by the Food and Drug Administration FDA or listed by the World Health Organization WHO for emergency use will be sufficient for travel. Americans vaccinated with the Janssen vaccine have a much stronger immune response to Covid-19 after a booster shot with an mRNA vaccine from Moderna or PfizerBioNTech than after a booster with Janssen. JNJ the Company today announced that the US. On April 23 CDC and the US.
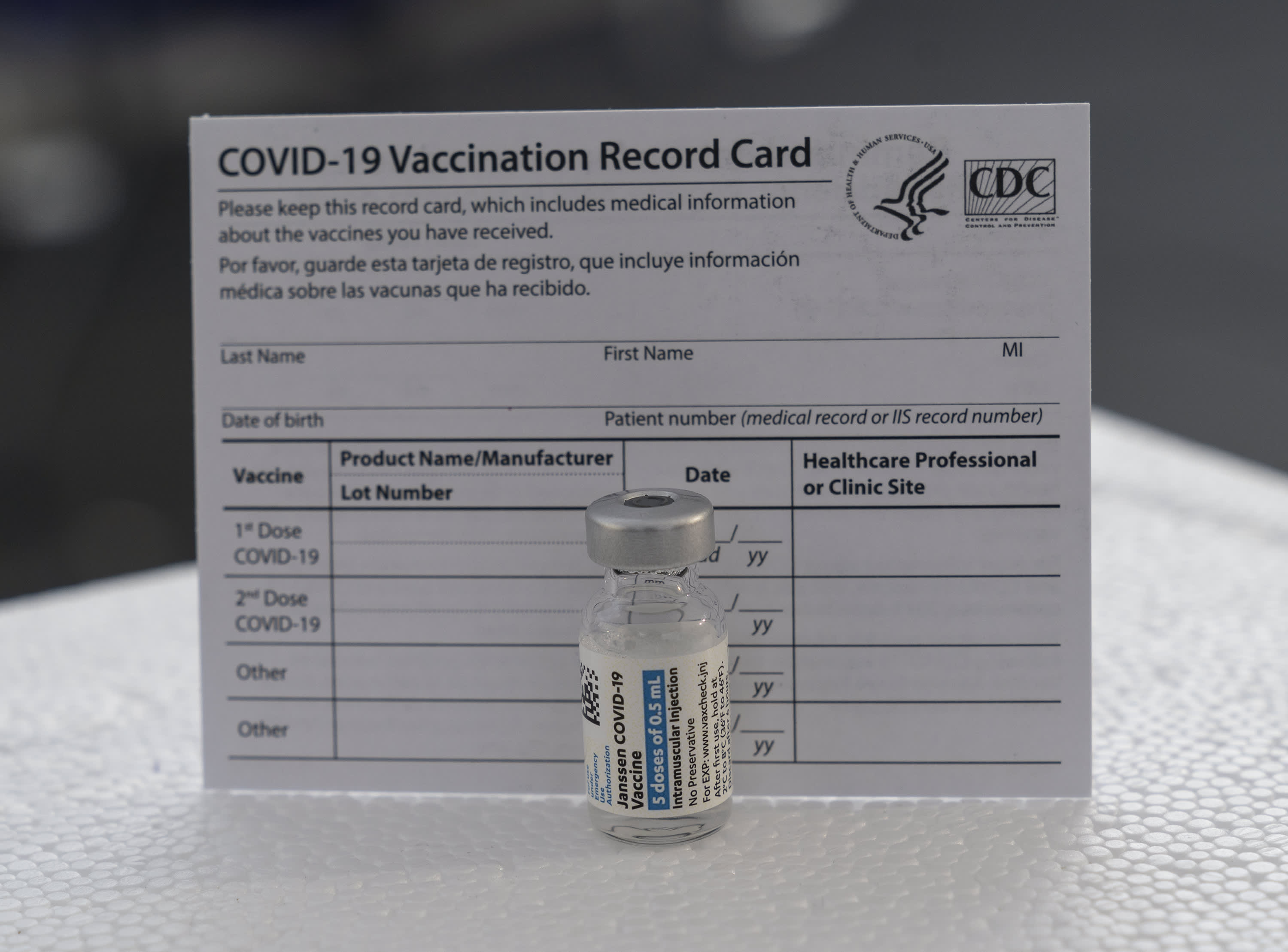
FDAs Vaccines and Related Biological Products Advisory Committee VRBPAC meeting the committee unanimously voted to recommend the Janssen COVID-19 Vaccine be Authorized for a second dose for certain people in the US. Although the Janssen vaccine had lower observed VE 1 dose of Janssen vaccine still reduced risk for COVID-19associated hospitalization by 71. It was formed when Johnson Johnson acquired the Dutch biotech company Crucell based in Leiden and placed it in their pharmaceutical division. Your Vaccination Card and Booster Shots At your first vaccination appointment you should have received a CDC COVID-19 Vaccination Record Card that tells you what COVID-19 vaccine you received the date you received it and where you received it.
What Are The Covid Vaccine Testing Requirements To Enter The US After Nov 8 2021. Food and Drug Administration FDA recommended use of Johnson Johnsons Janssen JJJanssen COVID-19 Vaccine resume in the United States after a temporary pause. In clinical trials more than 61000 individuals 18 years of age and older have receivedat least 1 dose of the Janssen COVID-19. For vaccines like Janssen Johnson Johnson which is also approved By FDA WHO you are considered fully vaccinated 2 weeks after you take the single dose which is the one and only dose for it.
Johnson and Johnsons vaccine Janssen was approved in late February and is the only single-shot vaccine available in the US. The donated vaccines will arrive in Bogota on July 1 and represent a. Janssen reported positive data from the Phase III UNIVERSE trial where the oral suspension formulation of Xarelto rivaroxaban was linked to reduced blood clots in paediatric Fontan procedure patients. Government of 25 million Janssen vaccines to the government of Colombia as part of the Biden-Harris Administrations worldwide efforts to fight the COVID-19 pandemic.
Any FDA-authorized or -approved vaccine can be used. Information about the JJJanssen COVID-19 vaccine including name manufacturer type of vaccine number of shots how it is given and links to ingredient information. Janssen Vaccines formerly Crucell is a biotechnology company specializing in vaccines and biopharmaceutical technologies. The JJJanssen COVID-19 vaccine has lower vaccine effectiveness over time compared to mRNA COVID-19 vaccines Pfizer-BioNTech and Moderna.
For use in individuals 18 years of age and older. The Janssen COVID-19 Vaccine has not been approved or licensed by the US. Reports after the use of JJJanssen COVID-19 Vaccine suggested an increased risk of a rare adverse event called thrombosis with thrombocytopenia syndrome TTS. Those vaccines include Pfizer Moderna AstraZeneca Covishield Sinopharm Sinovac and the one-shot Johnson Johnson jab.
The EUA is supported by a Secretary of Health and Human Services HHS. VE against COVID-19 hospitalization was slightly lower for the 2-dose Pfizer-BioNTech vaccine than the Moderna vaccine with this difference driven by a decline in VE after 120 days for the Pfizer-BioNTech but not the Moderna vaccine. Number of Vaccines Distributed by Company Janssen. The US Food and Drug Administration granted Breakthrough Therapy status for the vaccine in September 2019 to prevent LRTD caused by RSV in adults aged 60 years or above.
The FDA EUA Fact Sheet for Healthcare Providers Administering Vaccine Vaccination Providers and full EUA Prescribing Information are available here. The use of the vaccine was temporarily halted while reports of six. COVID-19 vaccines and other.

Panel Recommends J J Booster Cdc Issues Guidance On Future Vaccine Rollout For Ages 5 11 Coronavirus Update For Oct 19 2021 Cleveland Com
Johnson Johnson Vaccine U S Lifts Pause Coronavirus Updates Npr
U S Fda Advisers To Vote On J J Vaccine Booster Reuters
Australia Shelves Plans To Buy Johnson Johnson S Covid 19 Vaccine
What People Who Got J J Covid Vaccine Need To Know About Boosters
Statement By The Minister Of Health Dr Zwelini Mkhize On The Fda S Temporary Suspension Of Johnson And Johnson Vaccine Rollout In The United States Sahpra

Fda Orders Some 60 Million Johnson Johnson Vaccines Made By Emergent To Be Destroyed Health Policy Watch

U S Sends 1 Mln J J Covid 19 Vaccine Doses To S Korea State Dept Reuters
Posting Komentar untuk "Janssen Vaccine Usa"